Background Chemistry, Part 3
pH Value
The pH scale is used to express how acidic or basic a substance
is. Mathematically, pH is defined as the log10 [H3O+],
where [H3O+] is denoted for the concentration
of hydronium ions. Hydronium ion is the terminology for a proton that
is attached to a water molecule (H2O). When acids
dissolve or is diluted with water, it actually ionizes, or produces
ions. The protonated hydrogen from the acid is attracted to the water
molecules, forming the hydronium ion. From the concentration of hydronium
ions, [H3O+], we can use the pH scale to determine
the acidity of the new acid-water solution.
The pH scale has a range from 0 to 14. The lower the value of the pH,
the more acidic the solution is. The higher the pH, the more basic a
solution is. If a solution has a pH of 7, the solution is considered
neutral. Pure water is an example of a neutral solution. The tap water
you may drink at home, however, will be slightly higher or lower depending
on the ions it may contain.
Measuring pH
There are a few ways to measure pH such as using an indicator or a pH
meter.
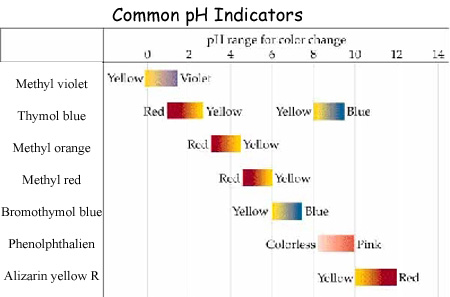
A simple way to measure pH is with indicators. Acid-base indicators
are substances that change color depending on the acidity or the alkalinity
(how basic) the solution is. A common acid-base indicator is phenolphthalein,
which produces a clear color in an acidic solution and will produce
a pink color in a basic solution. Methyl orange or methyl red are other
indicators that change color from shades of red to yellow. Litmus paper
is another type of indicator. Red litmus paper is used to identify bases,
by turning blue. Blue litmus paper, however, turns red when expose to
acids.
A pH meter can also be used to accurately measure pH down to a specific
pH value. This device is connected to a probe that is sensitive to H3O+
ions. There are manufacturers of pH probes including Vernier and Pasco.
Some probes may interface with graphing calculators and computers.
 To
Soil Conditioning
To
Soil Conditioning
